|
Population growth of a tropical tintinnid, Metacylis tropica on different temperature, salinity and diet |
|
Kyun-Woo Lee*, Young-Ung Choi Marine Life & Ecosystem Division, Korea Institute of Ocean Science & Technology
|
|
수온, 염분 및 먹이에 따른 열대 유종류, Metacylis tropica의 성장 |
|
이균우*, 최영웅 한국해양과학기술원
|
|
Abstract This study investigated the effects of temperature, salinity, and algal diet to find the optimal conditions for 5 days for the mass culture of the tropical tintinnid, Metacylis tropica. This tintinnid had a small, hyaline, and ovoid lorica. The oral diameter, length, and maximum width of the lorica were 36.7 μm, 49.5 μm, and 44.5 μm, respectively. In the temperature experiments, the highest maximum density and population growth rate were observed at 30°C with 340.7 cells/mL and 1.1/day, respectively. Lower salinities adversely affected the population growth of M. tropica. The maximum density was observed at 33 ppt (840 cells/mL). In the diet experiments, M. tropica fed Isochrysis galbana showed the highest density (413 cells/mL) and population growth rate (1.2/day). As a result, M. tropica is appropriate as a potential prey organism for early fish larvae with smaller mouths because the tintinnid has a relatively small size compared to the rotifer. In addition, the conditions of 30°C, 33 ppt and supplying I. galbana would be effective in the cultivation of M. tropica.
|
|
요 약 본 연구는 열대 유종류인 Metacylis tropica의 최적배양조건을 구명하기 위해 5일 동안 수온, 염분 및 먹이원인 미세조류의 효과를 조사하였다. M. tropica는 작고 투명한 난형의 피갑을 가지며, 피갑의 구강부 지름, 갑장 및 갑폭의 크기는 각각 36.7 μm, 49.5 μm 및 44.5 μm였다. 수온별 실험에서, M. tropica의 최고밀도와 성장률은 수온 30℃에서 각각 340.7 cells/mL와 1.1/day로 나타났다. 낮은 염분은 M. tropica의 성장에 악영향을 미쳤으며 염분 33 ppt에서 최고밀도가 840 cells/mL로 나타났다. 먹이실험에서, 먹이로 Isochrysis galbana를 공급하였을 때, 가장 높은 성장을 보였다(배양밀도, 413 cells/mL; 성장률, 1.2/day). 본 실험을 종합하여보면, M. tropica는 rotifer에 비해 작은 크기를 가지기 때문에 자어기에 입이 작은 어류의 초기먹이생물로 적합하며, 수온 30℃, 염분 33 ppt에서 I. galbana를 먹이로 공급하는 것이 M. tropica의 대량배양을 위한 최적배양조건인 것으로 판단된다.
Keywords : Diet, Metacylis tropica, Optimum culture condition, Salinity, Temperature, Tintinnid |
1. Introduction
Planktonic ciliate, tintinnids are important grazers of phytoplankton and are links between nanophyto- plankton and larval fish or crustaceans in marine food web[1]. The organisms are found in the gut of marine larval fish such as clupeids, gadids, flat fish, sciaenids, acanthurids and ammondytidae, particularly first- feeding larvae compared to old larvae prefer to feed on tintinnids[2]. In the study of Nagano et al. (2001), the aggregation of tintinnids in marine improved survival of first-feeding Japanese sand lance (Ammodytes personatus) larvae[3]. In addition, the survival of grouper (Epinephelus septemfasciatus) larvae was enhanced by presence of tintinnid as a live feed in laboratory study[4]. Thus, tintinnids have been considered promising candidates for mass production as main or additional live feeds for fish larvae in aquaculture[5]. However, little is known about the skill of mass culture of tintinnids.
We initially isolated the planktonic tintinnid, Metacylis tropica on a tropical coast located in Chuuk Lagoon, Micronesia near the equator. The tintinnid has relatively small size compared to rotifer (Brachionus rotundiformis, SS-type), provides advantages as being live food for marine fish larvae with small mouths[6]. Generally, suitable prey size to be ingested by larval fish is determined by larval mouth size[7].
The purpose of this study was to examine the effect of temperature, salinity and diet on the population growth of M. tropica to find optimum culture condition of the tintinnid which has never been reported before on its culture. In addition, we analyzed molecular taxonomy to identification of the tintinnid. These results provide basic information on the mass culture of the tintinnid which is used as initial livefeed for marine fish larvae with a small mouth.
2. Materials and Methods
2.1 Isolation, identification and
maintenance of the tintinnid
M. tropica used in this study were collected using plankton net (mesh size: 80 μm) in Chuuk Lagoon, Micronesia (7°27'07"N, 151°53'52"E) with temperature 29-32°C, salinity 30-33 ppt. The collected samples were carried rapidly to the laboratory in Korea-South Pacific Ocean Research Center, KIOST and isolated. Observations were made through the use of an inverted phase contrast microscope equipped with an ocular micrometer and a microphoto system at a magnification of 400X. Thirty individuals were fixed in 4% neutral buffered formalin solution and measured their sizes. The tintinnid was identified with morphological characteristics of lorica such as shape, length, maximal width and oral diameter[8].
For molecular taxonomy, total genomic DNA from the tintinnid was extracted using the DNeasy Blood & Tissue Kit (Qiagen). The partial sequence of 18S rDNA was determined using primers 18S-Tin3F: 5'- GCGGTATTTATTAGATAWCAGCC-3' and 28S-TinR1: 5'- TGGTGCACTAGTATCAAAGT-3' [9]. Polymerase chain reaction and DNA sequencing were performed using slightly modified methods described by Lee et al. (2014)[10]. The newly determined partial sequence was deposited in GenBank with accession number KP883283 and compared to GenBank database sequences. For species identification, nucleotide similarities with pairwise distance value were determined using MEGA 5.2[11].
Isolated M. tropica were cultured with the 250 mL Erlenmeyer flask contained 33 ppt filtered (0.2 μm) and autoclaved natural sea water. These were cultured with food mixture of Isochrysis galbana (~1×105 cells/mL) and Tetraselmis tetrathele (~2×104 cells/mL) at 30 ± 1°C.
2.2 Preparation of algal diets
The prymnesiophyte I. galbana (ISO: 3.2×5.3 μm), Green Isochrysis sp. (GISO: 3.7×3.5 μm), Pavlova lutheri (PAV: 5.5×3.7 μm), the prasinophyte Tetraselmis tetrathele (TET: 10.2×6.6 μm), Eustigmatophyte Nannochloropsis oculata (NAN: 2.6×2.6 μm), Synechococcus sp. (SYN: 1.1×1.2 μm), the diatom Chaetoceros simplex (CHA: 4.2×6.1 μm), were used as feed for the tintinnid. These algae were cultivated in Walne’s medium, using filtered (1μm mesh of glass fiber filter) and autoclaved seawater. The cultures were incubated at room temperature (20-23°C), under continuous light (4000 lx). Algae were fed to the tintinnid at the mid-to-late logarithmic phase.
2.3 The culture of the tintinnid
This study was divided into 3 parts: the effect of temperature, salinity and food. In all parts of experiments, M. tropica was inoculated at 3 cells/mL into 250 mL Erlenmeyer flask contained 150 mL of filtered (1 μm mesh of glass fiber filter) and autoclaved seawater. They were cultured under natural illumination and daily replenished I. galbana at 2×105 cells/mL for 5 days. Firstly, to investigate the effect of temperature, temperature was adjusted at 22, 26, 30 and 34°C and salinity was fixed at 33 ppt. The salinity effect was secondly investigated. The salinity was adjusted at 10, 15, 20, 25, 30 and 33 ppt and temperature was fixed at 30°C. These salinities were prepared by diluting seawater with distilled water. In the last experiment, the diet effect was investigated using mentioned seven microalgae. Before the test began, tintinnids were placed in the fresh medium containing each diet to remove the impact of diet history for 24 hours using 20 μm sieve. The volume of ISO (2×105 cells/mL) was used as standard to keep the equal biomass of diets in the vessel. Each treatment was replicated three times and every 12 hour recorded the number of tintinnid in 3 mL sub-samples under a stereomicroscope. Population growth rate (r) was calculated from following equation:
![]()
where, t is culture days with maximum density when tintinnid density (cell/mL) was the highest, Ni and Nt are the initial and highest tintinnid density, respectively.
2.4 Statistical analysis
Data were analyzed by one-way ANOVA. If significant (P<0.05) difference was found in the ANOVA test, Duncan's multiple range test was used to rank the groups. Data are presented as mean ± SE (standard error). All statistical analyses were conducted using SPSS program Ver. 12.0 (SPSS Inc., MichiganAvenue, Chicago, Illinois, USA)
3. Results and Discussion
3.1 Morphological characteristics and
molecular taxonomy of the tintinnid
The oral diameter, length and maximum width of the tintinnid used in this study were 36.7 ± 0.8 μm, 49.5 ± 5.5 μm and 44.5 ± 1.1 μm, respectively (n=30). The collar was short (7~8 μm) with 2 annuli (Fig. 1A). The shape of the aboral end was slightly pointed (Fig. 1B). According to the morphological characteristics of Durán (1957), the tintinnid was identified as M. tropica[8].
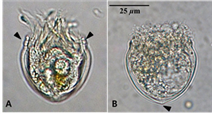
Fig. 1. Metacylis tropica. A, the focus is at the front of the tintinnid to find the annuli of collar (arrows indicate); B, fixed individual with formalin and an arrow indicate slightly pointed aboral end.
In addition, we analyzed the 18S rDNA gene to identify the tintinnid used in this study. The newly determined sequences (1490 bp long) were compared to GenBank sequences. The closest matches (99.6% similarity) in GenBank using BLAST search was the 18S rDNA sequence of M. angulata (GenBank accession numbers AY143568), followed by 99.4% similarity with M. pithos (JX101862).
3.2 The culture of the tintinnid
In the temperature experiments, the highest maximum density and population growth rate were found at 30°C with 340.7 cells/mL and 1.1/day (P<0.001), followed by 26°C with 131.7 cells/mL and 0.8/day respectively, but the treatment at 22°C kept low density with 1 to 7 cells/mL, even M. tropica at 34°C showed negative growth and eventually died on the 3.5thday (Table1, Fig. 2A).
The population growth of tintinnids is regulated by not only temperature but also food availability. According to Stoecker et al. (1983) and Verity (1985), the growth rates of Favella sp., Tintinnopsis vasculum and T. acuminate increased with increasing temperature [12,13]. In the present study, M. tropica showed a similar pattern to the above.
Lower salinities negatively affected the population growth of M. tropica. The maximum density was shown at 33 ppt (840 cells/mL), and significantly higher than others. While the population growth rate was the highest at 30 ppt (P<0.001), and the lowest at 20 ppt; they could not grow (Table 1, Fig. 2B). The maximum density and population growth rate of the tintinnid in this study were relatively higher than those of other tintinnid culture studies that were reported during the past several decades (Table 2).
Tintinnids are algivorous filter-feeder[4,14] but not all microalgae are used by the organisms. In this study, M. tropica fed ISO showed the highest density and population growth rate (413 cells/mL and 1.2/day, P<0.001). These results are similar to those of Graham and Strom (2010), who reported that non-toxic prey ISO support the growth of Metacylis sp.[15]. However, the tintinnid fed NAN died on day 3.5 (Table 1, Fig. 2C). It is known that ciliates are not able to digest phytoplankton with hard cell walls such as NAN because ciliates have no jaw which can break hard cell walls as a rotifer[16]. Picoplankton is important as a food source for microzooplankton, in practice, the cyanobacteria SYN is the most frequently observed food in the tintinnid vacuoles [17].
On the contrary, M. tropica showed the lowest population growth when they fed on SYN. This can be attributed to the toxicity of SYN or food size preference of M. tropica. It is known that SYN have the toxicity to Eutintinnus sp. and Metacylis sp.[18].
Table 1. Growth of Metacylis tropica cultured at different conditions
|
Experiments |
Maximum density (cells/mL) |
Population growth rate (/d) |
|
|
Temperature (°C) |
22 |
7.0 ± 0.58a |
0.4 ± 0.06a |
|
26 |
131.7 ± 5.36b |
0.8 ± 0.01a |
|
|
30 |
340.7 ± 31.20c |
1.1 ± 0.06b |
|
|
34 |
8.0 ± 2.65a |
0.6 ± 0.20a |
|
|
Salinity (ppt) |
10 |
-1 |
- |
|
15 |
- |
- |
|
|
20 |
5.3 ± 0.33a |
0.4 ± 0.13a |
|
|
25 |
183.3 ± 25.10b |
1.2 ± 0.08b |
|
|
30 |
596.7 ± 29.63c |
1.7 ± 0.08c |
|
|
33 |
840.0 ± 46.19d |
1.4 ± 0.12bc |
|
|
Diet |
ISO |
413.3 ± 48.48c |
1.2 ± 0.06c |
|
TET |
129.3 ± 42.73b |
0.8 ± 0.06b |
|
|
GISO |
84.7 ± 14.81ab |
0.9 ± 0.02b |
|
|
PAV |
45.3 ± 11.68ab |
1.0 ± 0.01b |
|
|
CHA |
5.3 ± 0.33a |
0.5 ± 0.10a |
|
|
NAN |
- |
- |
|
|
SYN |
4.3 ± 0.33a |
0.5 ± 0.09a |
|
1Negative population growth rate.
ISO, Isochrysis galbana; GISO, Green Isochrysis sp.; PAV, Pavlova lutheri; TET Tetraselmis tetrathele; NAN, Nannochloropsis oculata SYN, Synechococcus sp.; CHA, Chaetoceros simplex.
a,b,cValues (Mean±SE) within a column with different superscript letters are significantly different (P<0.05).
On the other hand, tintinnids are known as a selective filter feeder based on prey size[17]. In case of the genus Favella, the organism tends to have a preference for larger sized prey. In the study of Stoecker et al. (1995), 10 μm particles (equivalent spherical diameter) were captured more efficiently than smaller particles (4 μm) by Favella sp.[19]. Moreover, Kamiyama and Arima (2001) reported that the prey selectivity of F. taraikaensis is higher for larger algae than the smaller algae such as PAV under mixed prey conditions[14]. Preferred prey size is related to lorica oral diameter[20] and the maximum ingested food size is up to 40-45% of the oral diameter of the lorica in tintinnids (Spittler 1973). Therefore, M. tropica can ingest the prey with the maximum size of 16.5 μm in this study. In the case of CHA, it has the suitable size of 4.2×6.1 μm to be ingested, while it would be difficult to be taken by M. tropica due its long seta (approximately 24 μm) which is over the size limit.
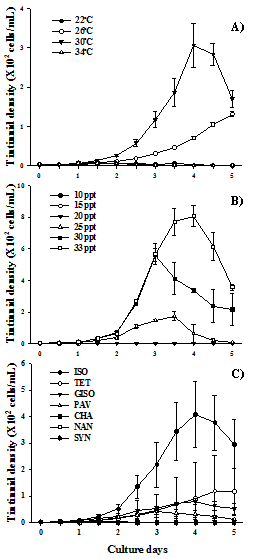
Fig. 2. Variation of population growth against temperature (A), salinity (B) and diet (C) in the tintinnid, Metacylis tropica. Vertical bars describe standard error.
Despite GISO and PAV have similar size to ISO, the growth of M. tropica fed the two diets were lower than that of the tintinnid fed ISO. This may be due to the difference of prey quality such as nutrition, digestibility and so on. Further study is required to understand the cause of the difference.
Table 2. Growth rates (GR) and maximum densities (MD) of tintinnids in laboratory experiments
|
Species |
GR (/d) |
MD (ind./mL) |
Diet |
Temp. (°C) |
Source |
|
Favella sp. |
1.39 |
|
Heterocapsa triquetra |
20 |
[12] |
|
T. acuminata |
2.00b |
<10 |
I. galbana |
20 |
[13] |
|
T. vasculum |
1.10b |
<10 |
Dicrateria inornata |
15 |
[13] |
|
T. beroidea |
~0.46a |
720 |
Gymnodinium sp., Paviova lutheri |
22 |
[22] |
|
Amphorella quadrilineata |
~0.46a |
437 |
Gymnodinium sp., P. lutheri |
22 |
[22] |
|
F. taraikaensis |
1.19 |
|
Alexandrium tamarense |
15 |
[23] |
|
F. taraikaensis |
1.31 |
|
H. triquetra |
15 |
[23] |
|
Metacylis tropica |
1.70 |
840 |
I. galbana |
30 |
present study |
a Estimated from figure of sources. bRecalculated from the value of source by the equation used in present study.
4. Conclusion
The conditions of 30°C, 33 ppt and supplying I. galbana would be effective in the cultivation of M. tropica. These results indicate that M. tropica is appropriate for being a test organism and a potential prey organism for early fish larvae with smaller mouths. Further studies including the effects of additional physical and biological factors are required to establish a stable method of mass culturing the tintinnid.
References
[1] J. R. Dolan, “Introduction to tintinnids”, in The biology and ecology of tintinnid ciliates: models for marine plankton, edited by John R. Dolan, David J.S. Montagnes, Sabine Agatha, D. Wayne Coats, and Diane K. Stoecker (John Wiley & Sons, Ltd., Chichester, 2013), pp. 10-28.
[2] D. K. Stoecker, “Predators of tintinnids”, in The biology and ecology of tintinnid ciliates: models for marine plankton, edited by John R. Dolan, David J.S. Montagnes, Sabine Agatha, D. Wayne Coats, and Diane K. Stoecker (Johb Wiley& Sons, Ltd., Chichester, 2013), pp. 122-144.
[3] N. Nagano, Y. Iwatsuki, Y. Okazaki, and H. Nakata, “Feeding strategy of Japanese sand lance larvae in relation to ciliated protozoa in the vicinity of a thermohaline front”, Journal of Oceanography 57, 155-163. 2001.
DOI: http://dx.doi.org/10.1023/A:1011139107186
[4] N. Nagano, Y. Iwatsuki, T. Kamiyama, and H. Nakata, “Effects of marine ciliates on survivability of the first-feeding larval surgeonfish, Paracanthurus hepatus: laboratory rearing experiments”, Hydrobiologia 432, 149-157. 2000.
DOI: http://dx.doi.org/10.1023/A:1004094825739
[5] G. D. Treece and D. A. Davis, “Culture of small zooplankters for the feeding of larval fish”, SRAC Publication No. 701 2000.
[6] A. Hagiwara, W. G. Gallardo, M. Assavaaree, T. Kotani, and A. B. de Araujo, “Live food production in Japan: recent progress and future aspects”, Aquaculture 200, 111-127. 2001.
DOI: http://dx.doi.org/10.1016/S0044-8486(01)00696-2
[7] I. Cunha and M. Planas, “Optimal prey size for early turbot larvae (Scophthalmus maximus L.) based on mouth and ingested prey size”, Aquaculture 175, 103-110. 1999.
DOI: http://dx.doi.org/10.1016/S0044-8486(99)00040-X
[8] M. Durán, “Nota sobre algunos tintinnoineos del plancton de Puerto Rico”, Invest Pesq Tomo VIII, 97-120. 1957.
[9] C. Bachy, F. Gomez, P. Lopez-Garcia, J. R. Dolan, and D. Moreira, “Molecular phylogeny of tintinnid ciliates (Tintinnida, Ciliophora)”, Protist 163, 873-887. 2012.
DOI: http://dx.doi.org/10.1016/j.protis.2012.01.001
[10] K. W. Lee, J. H. Kang, S. H. Baek, Y. U. Choi, D. W. Lee, and H. S. Park, “Toxicity of the dinoflagellate Gambierdiscus sp. toward the marine copepod Tigriopus japonicus”, Harmful Algae 37, 62-67. 2014.
DOI: http://dx.doi.org/10.1016/j.hal.2014.05.007
[11] K. Tamura, D. Peterson, N. Peterson, G. Stecher, M. Nei, and S. Kumar, “MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods”, Mol. Biol. Evol. 28, 2731-2739. 2011.
DOI: http://dx.doi.org/10.1093/molbev/msr121
[12] D. Stoecker, L. H. Davis, and A. Provan, “Growth of Favella sp. (Ciliata, Tintinnina) and other microzooplankters in cages incubated in situ and comparison to growth in vitro”, Mar. Biol. 75, 293-302. 1983.
DOI: http://dx.doi.org/10.1007/BF00406015
[13] P. G. Verity, “Grazing, respiration, excretion, and growth rates of tintinnids”, Limnol. Oceanogr. 30, 1268-1282. 1985.
DOI: http://dx.doi.org/10.4319/lo.1985.30.6.1268
[14] T. Kamiyama and S. Arima, “Feeding characteristics of two tintinnid ciliate species on phytoplankton including harmful species: effects of prey size on ingestion rates and selectivity”, J. Exp. Mar. Biol. Ecol. 257, 281-296. 2001.
DOI: http://dx.doi.org/10.1016/S0022-0981(00)00341-5
[15] S. L. Graham and S. L. Strom, “Growth and grazing of microzooplankton in response to the harmful alga Heterosigma akashiwo in prey mixtures”, Aquat. Microb. Ecol. 59, 111-124. 2010.
DOI: http://dx.doi.org/10.3354/ame01391
[16] S. H. Cheng, S. Aoki, M. Maeda, and A. Hino, “Competition between the rotifer Brachionus rotundiformis and the ciliate Euplotes vannus fed on two different algae”, Aquaculture 241, 331-343. 2004.
DOI: http://dx.doi.org/10.1016/j.aquaculture.2004.08.006
[17] P. Pitta, A. Giannakourou, and U. Christaki, “Planktonic ciliates in the oligotrophic Mediterranean Sea: longitudinal trends of standing stocks, distributions and analysis of food vacuole contents”, Aquat. Microb. Ecol. 24, 297-311. 2001.
DOI: http://dx.doi.org/10.3354/ame024297
[18] D. J. S. Montagnes, “Ecophysiology and behavior of tintinnids”, in The biology and ecology of tintinnid ciliates: models for marine plankton, edited by John R. Dolan, David J.S. Montagnes, Sabine Agatha, D. Wayne Coats, and Diane K. Stoecker (John Wiley & Sons, Ltd., 2013).
[19] D. K. Stoecker, S. M. Gallager, C. J. Langdon, and L. H. Davis, “Particle capture by Favella sp. (Ciliata, Tintinnina)”, J. Plankton. Res. 17, 1105-1124. 1995.
DOI: http://dx.doi.org/10.1093/plankt/17.5.1105
[20] J. R. Dolan, “Morphology and ecology in tintinnid ciliates of the marine plankton: correlates of lorica dimensions”, Acta Protozool. 49, 235-244. 2010.
[21] Y.-O. Kim, E. J. Yang, J.-H. Kang, K. Shin, M. Chang, and C. S. Myung, “Effects of an Artificial Breakwater on the Distributions of Planktonic Microbial Communities”, Ocean Sci. J. 42, 9-17. 2007.
DOI: http://dx.doi.org/10.1007/BF03020906
[22] T. Kamiyama and Y. Aizawa, “Growth characteristics of two tintinnid ciliates, Tintinnopsis beroidea and Amphorella quadrilineata, in laboratory cultures”, Bull. Plankton Soc. Japan 34, 185-191. 1987.
[23] T. Kamiyama, M. Tsujino, Y. Matsuyama, and T. Uchida, “Growth and grazing rates of the tintinnid ciliate Favella taraikaensis on the toxic dinoflagellate Alexandrium tamarense”, Mar. Biol. 147, 989-997. 2005.
DOI: http://dx.doi.org/10.1007/s00227-005-1629-2
|
이 균 우(Kyun-Woo Lee) [정회원] |
|
|
|
•2001년 2월 : 강릉원주대학교 해양생명공학과 (이학석사) •2004년 2월 : 강릉원주대학교 해양생명공학과 (이학박사) •2013년 8월 ~ 현재 : 한국해양과학기술원 선임연구원 |
|
<관심분야> 해양생물학, 해양환경독성학 |
|
|
최 영 웅(Young-Ung Choi) [정회원] |
|
|
|
•2002년 2월 : 제주대학교 수산생물학과 (이학석사) •2006년 8월 : 제주대학교 수산생물학과 (이학박사) •2012년 7월 ~ 현재 : 한국해양과학기술원 책임연구원 |
|
<관심분야> 해양생물학, 번식생리학 |
|

